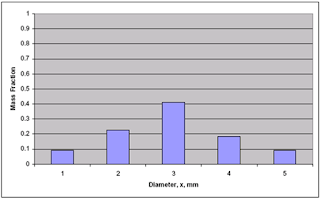
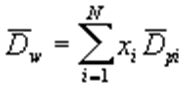Size measurement with fine particles
- Dry screening is useful for sizing particles with diameter greater than about 44 μm (325 mesh).
- Wet screen analysis can be used for diameters down to 10 μm.
- Optical microscopy and gravity sedimentation are used with particles 1 – 100 μm.
- Coulter counter, a device used for sizing and measuring particles by measuring change in resistivity of an electrolyte as it carry particle one by one through a small orifice.
- Light scattering techniques, sedimentation in centrifuges and electron microscopy are other useful method for measuring size of even smaller particles.
Particulate Solids In Bulk
- Masses of solid particles, especially when they are dry and not sticky, have many properties of a fluid.
- They exert pressure on sides of walls of container.
- They flow through opening or inclined plane / channel.
- Depending upon the flow property particulate solids are divided into two classes, cohesive (wet clay, reluctant to flow through opening) and non-cohesive (grains, dry sand, plastic chips etc readily flow out of bin or silo).
- They differ from liquid and gasses in several ways because of particles interlocked at high pressure.
- Before the mass of tightly packed particles can flow, it must increased in volume to permit interlocking grains to move past one another.
Voidage
- Voidage is the fraction of the total volume which is made up of the free space between the particles and is filled with fluid.
- One of the most important characteristics of any particulate mass.
- Voidage is the fraction of the total volume which is made up of the free space between the particles and is filled with fluid.
- Voidage corresponds to density of packing of the particles.
- In general, isometric particles, will pack more densely than long thin particles or plates.
- The more rapidly material is poured on to a surface or into a vessel, the more densely will it pack.
- If it is then subjected to vibration, further consolidation may occur.
- The packing density or voidage is important in that it determines the bulk density of the material.
- It affects the tendency for agglomeration of the particles.
- It critically influences the resistance offers to the fluid flowing through it as for example in filtration.
Agglomeration
- Agglomeration arises from interaction between particles, as a result of which they adhere to one another to form clusters.
- The main mechanisms giving rise to agglomeration are:
- Mechanical interlocking: This can occur particularly if the particles are long and thin in shape, in which case large masses may become completely interlocked.
- Surface attraction: Surface forces, including van der Waals’ forces, may give rise to substantial bonds between particles, particularly where particles are very fine (<10 μm), with the result that their surface per unit volume is high. In general, freshly formed surface, such as that resulting from particle fracture, gives rise to high surface forces.
- Plastic welding: When irregular particles are in contact, the forces between the particles will be applied on extremely small surfaces and the very high pressures developed may give rise to plastic welding.
- Electrostatic attraction: Particles may become charged as they are fed into equipment and significant electrostatic charges may be built up, particularly on fine solids.
- Effect of moisture: Moisture may have two effects. Firstly, it will tend to collect near the points of contact between particles and give rise to surface tension effects. Secondly, it may dissolve a little of the solid, which then acts as a bonding agent on subsequent evaporation.
- Temperature fluctuations: give rise to changes in particle structure and to greater cohesiveness.
Pressure in particulate solids
- The exerted pressure is not same in all directions. In general the pressure applied in one direction creates some pressure in other directions.
- The minimum pressure in solid masses is in the direction normal to that of applied pressure.
- In homogenous masses the ratio of normal pressure to applied pressure is constant which is the characteristic of material which depends on:
- shape and interlocking tendency of particles,
- stickiness of grain surfaces,
- and degree of packing.
- It is nearly independent of particle size until the grain become very small and material is no loner free-flowing.
Angle of repose
- When the granular solid are piled up on a flat surface, the sides of the pile are at a definite reproducible angle with the horizontal. This angle is called angle of repose of that material.
- If solid is poured from a nozzle on to a plane surface, it will form an approximately conical heap and the angle between the sloping side of the cone and the horizontal is the angle of repose. When this is determined in this manner it is sometimes referred to as the dynamic angle of repose or the poured angle.
- The angle of repose may also be measured using a plane sheet to which is stuck a layer of particles from the powder. Loose powder is then poured on to the sheet which is then tilted until the powder slides. The angle of slide is known as the static angle of repose or the drained angle.
- Angles of repose vary from about 20◦ with free-flowing solids, to about 60◦ with solids with poor flow characteristics.
- Powders with low angles of repose tend to pack rapidly to give a high packing density.
- An angle which is similar to the static angle of repose is the angle of slide which is measured in the same manner as the drained angle except that the surface is smooth and is not coated with a layer of particles.
- A measure of the frictional forces within the particulate mass is the angle of friction.

- The angle of friction is important in its effect on design of bin and hoppers.
- If the pressure at the base of a column of solids is measured as a function of depth, it is found to increase approximately linearly with height up to a certain critical point beyond which it remains constant.

- For heights greater than Lc the mass of additional solids is supported by frictional forces at the walls of the hopper.
Coarse solid like gravel, sand and coal are stored outside in large pile unprotected from weather.
Solids that are two valuable and soluble on expose to outdoor piles are stored in bins, hoppers or silos.
- When hundred and thousands of tons of solids are involved then storing out door in a pile is the most economical method.
- Valuable solids are stored in bins, hoppers or silos.
- These are cylindrical or rectangular vessel of concrete or metal.
- Silo is tall relatively small in diameter.
- Bin is not very tall but fairly wide.
- Hopper is small vessel with sloping bottom.
- Silos and bins are used storage for some period of time while hoppers are used for temporary storage before feeding solid to the process.
- All these container are loaded from top by some kind of elevator; discharging is from the bottom.
- The major problem in solid storage vessel design is to provide satisfactory discharge.
Flow of solids in hoppers
- Discharge from the hopper takes place through an aperture at the bottom of the cone, and difficulties are commonly experienced in obtaining a regular, or sometimes, any flow.
- Commonly experienced types of behavior are shown in Figure 1.15.
- Bridging of particles may take place and sometimes stable arches (b) may form inside the hopper. These can usually be broken down by vibrators attached to the walls.
- A further problem which is commonly encountered is that of “piping” or “rat-holing”(c), in which the central core of material is discharged leaving a stagnant surrounding mass of solids. As a result some solids may be retained for long periods in the hopper and may deteriorate.
- Ideally, “mass flow” (a) is required in which the solids are in plug flow and move downwards in masse in the hopper. The residence time of all particles in the hopper will then be the same.
- In general, tall thin hoppers give better flow characteristics than short wide ones and the use of long small-angle conical sections at the base is advantageous.
- The nature of the surface of the hopper is important and smooth surfaces give improved discharge characteristics.
Discharge rate, measurement and control of solids flowrate